
Transmune® stops reinfection and protects against all IBD virus strains

The vaccine
Transmune® is an IBD immune complex vaccine consisting of the Winterfield 2512 strain linked to specific antibodies called Virus Protecting Immunoglobulins, to be administered at the hatchery


The product was developed in the early 2000s, and quickly registered in many countries around the world. Currently, the vaccine is marketed in over 75 countries worldwide.
How Immune Complex works
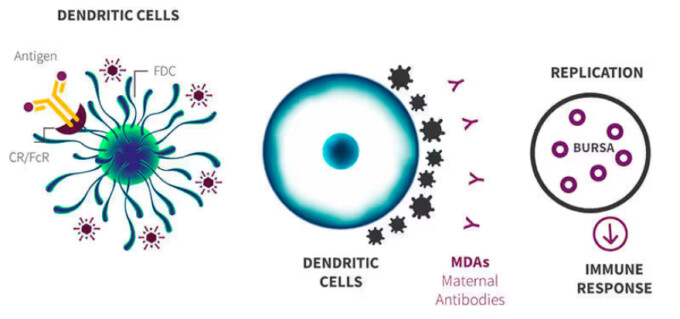

Stop the gumboro cycle
Peace of mind
Stops the Gumboro cycle
Consistency
Flocks with uniform results
Trust
Strong partnership between producers and Ceva year after year

PEACE OF MIND through stopping the Gumboro Cycle
Strain isolation (%) Between different vaccination programs in 4 year-period (2013-2016), in 527 flocks.
Transmune® Consistently blocks the bursa against others IBD strains.

CONSISTENCY of uniform titers
Transmune®
Mean titer of IBD in flocks vaccinated with Transmune®. Total samples: 702 flocks.

Drinking water
Mean titer of IBD in flocks vaccinated with vaccines by DW. Total samples: 336 flocks.

rHVT-VP2
Mean titer of IBD in flocks vaccinated with rHVT-VP2. Total samples: 134 flocks.

TRUST through high performance


The vaccine
CID 50 test
Since Transmune is registered in Europe a unique QC procedure had to be developed to safeguard the efficacy and safety of the vaccine.
Every single production batch is thoroughly tested using a CID (Chick Infective Dose 50) test. This test is used with the final blended product to guarantee the potency and safety of the vaccine.


Our Services
IBD CONTROL FROM THE HATCHERY
Gumboro disease, also known as Infectious Bursal Disease (IBD), is a highly contagious disease in young chickens caused by the Infectious Bursal Disease Virus (IBDV).

 Poultry
Poultry


